Hard Water
Learn exactly what hard water is, how it’s created and what minerals it is composed of.

65% of UK Mains Water is Designated Hard
Causes Limescale in Pipes & Appliances
1mm of Scale Increases Energy Costs by Over 7%
The ACP leaves essential
minerals in water unchanged
Chemistry of Hard Water
Water is said to be hard if it contains high levels of certain minerals such as Calcium (Ca) and Magnesium (Mg). When hard water is heated these minerals combine with carbon compounds to form hard water scale often called Scale, Limescale or Calcite. The chemical name for this is Calcium Carbonate which has the formula CaCO3.

Calcite Scale
Why Does Hard Water Occur?
When rain falls it dissolves Carbon Dioxide (CO2) in the air making the rain acidic. This rain dissolves minerals in certain types of rock, mainly sedimentary rock such limestone which creates hard water. Igneous rock such as granite is resistant to acid rain hence no minerals are dissolved. Water from areas of igneous rock tends to be soft. Hence water hardness is more to do with the local rock type rather than the water itself.



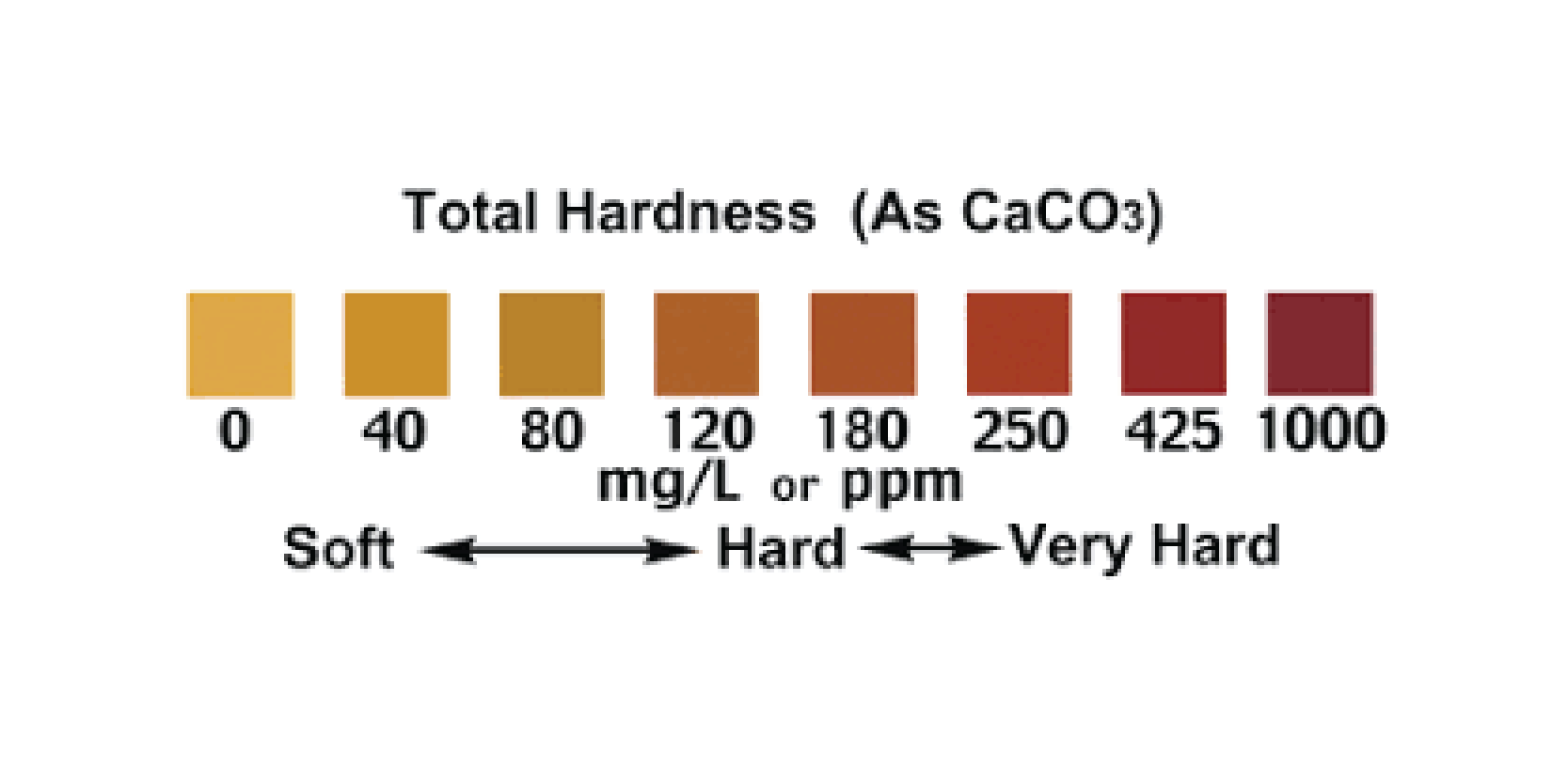
How is Water Hardness Measured?
The major component of hard water is Calcium (Ca).
While Magnesium (Mg) will be present at 15 – 20% that of Ca other minerals/ions represent < 1% of the concentration of Ca.
Hard water can be presented as Total Hardness which represents the concentration of all dissolved ions expressed as mg/l or ppm Calcium Carbonate (CaCO3).
These are expressed as mg/l of dissolved substance ie 150mg/l CaCO3 is 150 mg of CaCO3 in 1 litre of water.
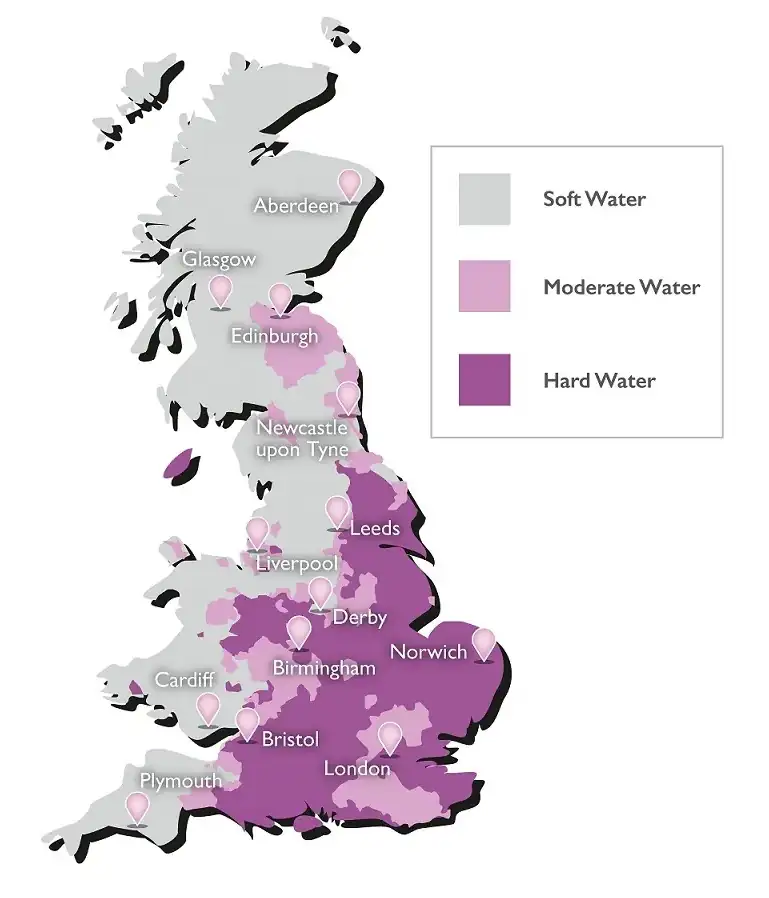
Hard Water Areas Within the UK
The hard water map of the British mainland shows that the majority of the UK population live in hard water or very hard water areas. Hard water is defined by the levels or concentration of minerals in it. Predominantly Calcium (Ca) and Magnesium (Mg) these minerals are important to diet and health.
Where’s your business and where do you live? If you live or work in any of the shaded areas limescale will be costing you money.
Well over 60% of water supplied in the UK is moderately hard or above. When hard water is heated it forms calcite limescale which blocks pipes, increases energy bills, reduces equipment life and increases the need for maintenance. Not to mention the unsightly, hard to remove scale which forms around hot water outlets. If you live in a hard water area and are not treating your water you will be paying significantly higher energy bills and suffering a higher level of equipment failure than those that do.
What is Considered to be Hard Water?
The following table gives an indication of Soft to Very Hard Water values.
Classification
Soft
Medium
Hard
Very Hard
Level
Less than 100 mg/L
101-150 mg/L
151-300 mg/L
Great than 301 mg/L
Energy Wastage Caused by Limescale
Calcite limescale is a poor conductor of heat. Scale that forms inside pipework requires more heat which increases energy bills. The Carbon Trust have published that 1mm of scale will increase energy demand by 7%. In hard water areas this will form in the first year. Subsequent cumulative energy losses due to scale are likely to be 12% in year 2 and 16% in year 3.
| Annual Energy Costs | Increased Energy Costs Due to Limescale | Cumulative Savings Over 3 Years |
||
|---|---|---|---|---|
| 1 Year | 2 Years | 3 Years | ||
| Small House (£1.5k) | £1,605 | £1,680 | £1,725 | £510 |
| Large House (£3k) | £3,210 | £3,360 | £3,450 | £1,020 |
| Small Commercial (£10k) | £10,700 | £11,200 | £11,500 | £3,400 |
| Large Commercial (£40k) | £42,800 | £44,800 | £46,000 | £13,600 |
Examples of Limescale Damage
Limescale builds up because of hard water, which contains high concentrations of dissolved minerals like calcium and magnesium. When this water evaporates, these minerals are left behind and crystallize, forming a white, chalky deposit called limescale. Heating the water accelerates this process, as heat causes the minerals to more readily form deposits on surfaces.
How is Limescale Formed?
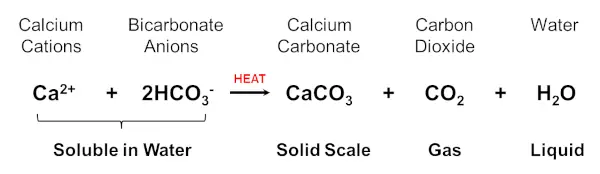
The main problem with hard water is the build-up of Limescale (CaCO3) which is formed when hard water is heated.
Hard water contains soluble Calcium Hydrogen Carbonate, Ca(HCO3)2 which when heated converts to CaCO3. This drops out of solution as a hard solid and adheres strongly to metal, plastic or wood.
This is shown by the following chemical equation:
Without treatment the vast majority of Scale forms as Calcite. This is a hard, brittle scale which adheres strongly to heated surfaces. It has very poor thermal conductivity, restricts flow, contributes to higher maintenance needs and reduces equipment life.
CALCITE scale also forms a safe breeding ground for bacteria and microorganisms.
Need an experts opinion?
What is the Cost of Hard Water?
Increase energy cost due to heat losses from scale::
- 1.5 mm of scale increases energy costs by 12%.
- 5 mm adds 38% to the energy costs.
- An estimated £1Bn in energy losses in the UK due to hard water scale.
- Cost of additional system maintenance.
- Reduced equipment life and early replacement cost.
- Flow restriction reducing system efficiency.
- Scale encourages microbial activity and all the cost implications thereof.
Without treatment any system will have a significantly shorter lifespan and a considerably more expensive one.
UK professional bodies strongly recommend hard water scale treatment.
Such treatment will keep a building system clean, less prone to equipment failure, reduced need for maintenance and use significantly less energy lowering both costs and environmental impact.
As a guide for a new build/system reducing scale will save 50% of the annual energy cost during the first 3 years.












